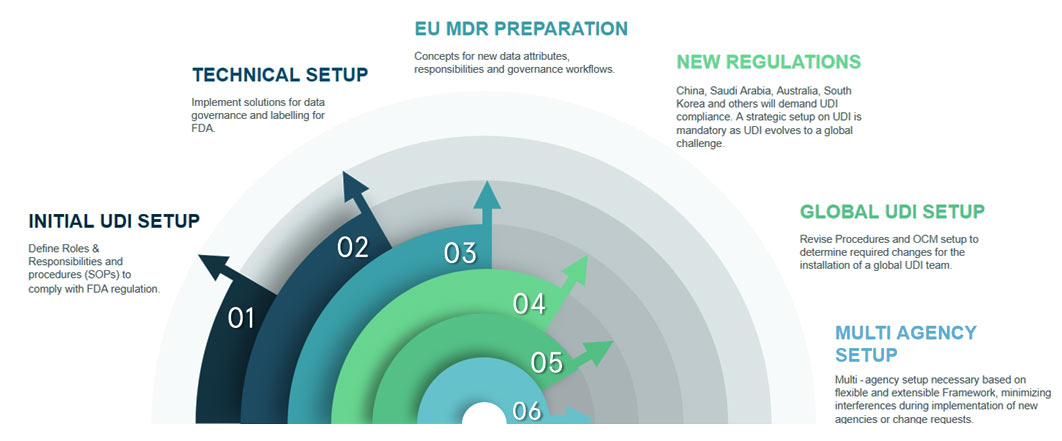
On September 24, 2013, the U.S. Food and Drug Administration (FDA) announced the new requirement to identify medical devices that affect its safe and effective use through the use of UDI.
Medical Device manufacturer companies have to create and maintain the Unique Device Identifier (UDI) which is specific to a device model, and a production identifier, which includes the current production information for that specific device, such as the lot or batch number, the serial number and/or expiration date.
UDI will be a unique numeric or alphanumeric code consisting of two parts, DI – identifies the labeler and the specific version or model of the device and PI – variable portion of UDI which is combination of lot/batch number, serial number etc.